
(c) What should be the drum temperature if we want 50% of the feed to evaporate in the flash drum? Find the corresponding changes in x and y. It possible to get a vapor product containing 80 mole% ethanol from this unit? (b) If the drum temperature is 85 oC, find x, y,VF Test your understanding: Problem 1 A flash distillation chamber operating at 1 atm (101.3 kPa) is separating ethanol-water mixture containing 60 mole % ethanol.

Graphical simple and insightful Slope of eq (6a) L 1 fĬondition to prevent boiling at TF i i F F ii i Numerical General method including multi-componentĪnalytical Equation for equilibrium data, e.g., eq (4) Overall mass balance F V L (5) Component balance (more volatile species) Internal enthalpy supply causes Tdrum < TF Valve causes part of the feed to flash from the liquid to the vapor state. Pressure reduction from PF to Pdrum across the TF and PF TF from energy balance around Loop 1Įmpirical method depends only on densities and mass flow rates of L and VĪdiabatic process. Overall and component mass balances around Loop 1 x, y, V Given Calculate How? Tdrum, Pdrum x, y, V What do we mean by designing a flash system? Remember: x and y are in equilibrium T, P, xA and yA are the 4 independent variables Condense a vapor mixture to obtain a desired liquid mixture. From a given liquid mixture, obtain a specific vapor mixture at specified temperature and pressure. A binary vapor-liquid system is at 50oC and 1 atm pressure. To equilibrium with a 70:30 vapor mixture of A:B at 70oC. TfKTfK BA TfABissystemsmanyforassumptiongoodAĭegrees of freedom in a binary two-phase systemį: degrees of freedom C: number of components P: number of phase Test your understanding You are asked to determine the system pressure that is required to bring a 50:50 liquid mixture of A:B to come
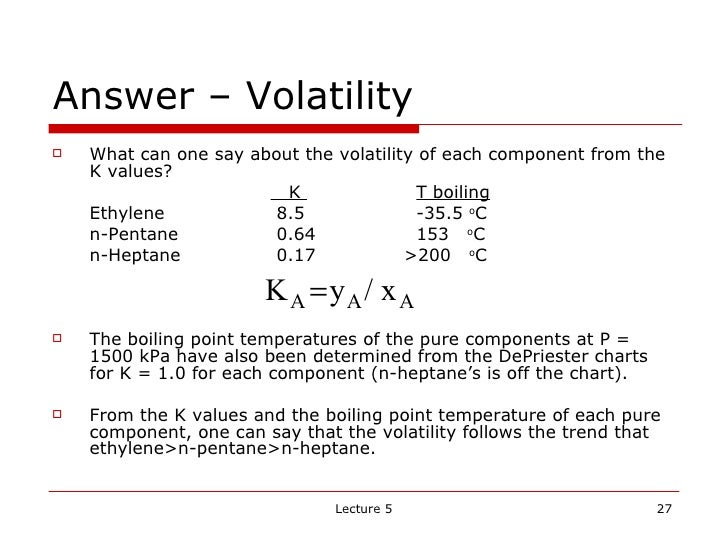
If no, xA = xA + x and go to (iv)Īpproximate equilibrium equation for a binary system Calculate KA and KB from Eqs (1) and (2) or from DePriester Chart x = 0.1 (depends on how many data points) iii.
Depriester chart to read boiling point how to#
How to generate x-y-T data from Ki values Illustration for a binary system Tables of pure component vapor pressures are also available. These are tabulated in various data sources. I, where The following identities apply for both ideal and non-ideal systemsīAp ln (2) Ai, Bi, Ci are constants for pure components. Vapor-liquid equilibrium for an ideal system Read K values methane, ethylene, iso-propane and n-octane at 200 kPa and 50 oC The diagonal joins (0, 0) and (1,1) points and is an important reference lineĭePriester chart: K values for light hydrocarbons This is Figure 2-12 from the text book. X-y diagram usually scales from 0-1 on both axes
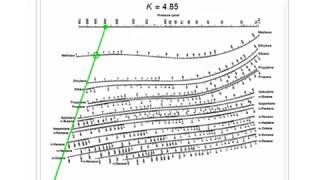
X-y diagram is widely used in binary vapor-liquid Models relating y to x at equilibrium were covered in CN2121 Simple equations for ideal systems Complex equations for non-deal systemsĭesign of the flash process depends on good equilibrium dataĭePriester chart for hydrocarbons Simple thermodynamic model forĮxperimental vapor-liquid equilibrium data (including non-ideal systems) Tabular (complete with temperature and pressure)
Depriester chart to read boiling point tv#
V and L are in equilibrium TL = TV = Tdrum Liquid and vapor stream pressures = Pdrum y= f(x) yi / xi= Ki (distribution coefficient) = f(P, T and all xi) xA + xB = 1 = yA + yB What can we say from the equilibrium design method? Vapor pressure of A: pA Vapor pressure of B: pBĭemisterDemister prevents liquid droplet entrained in vapor Concept of phase equilibrium Equilibrium relationshipsįlash distillation Design issues Binary flash distillation design Problem to test your understandingĬompulsory reading: Chapter 2 (sections 2.1 to 2.3, 2.4, 2.4.1, and 2.5) from the text bookįlash Distillation simplest separation process in chemical industryįeed is a liquid mixture Methanol (A) water (B) Propane (A) butane (B) Water (A) salt (B)


 0 kommentar(er)
0 kommentar(er)
